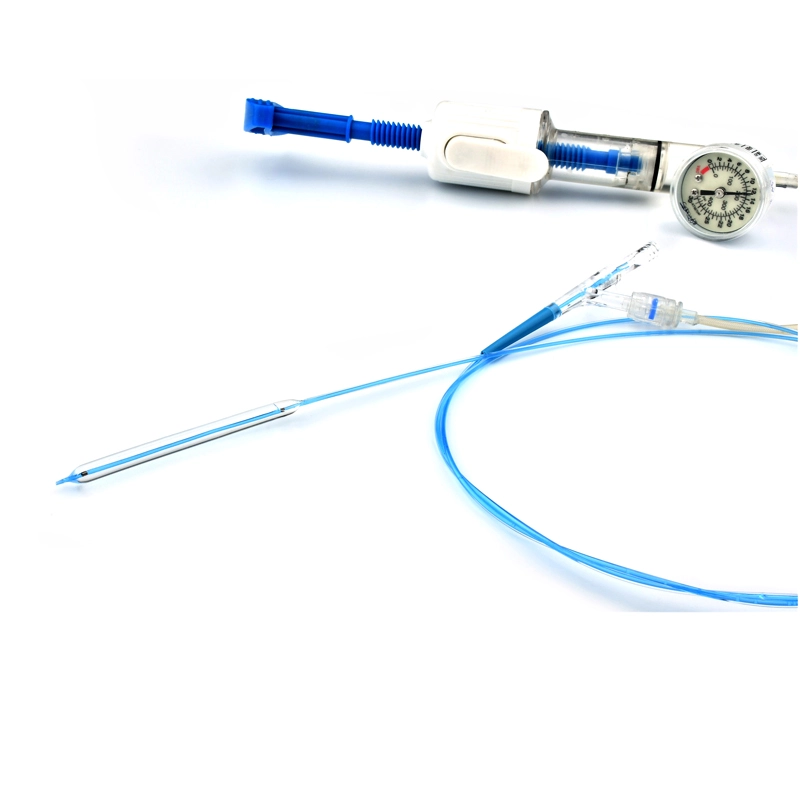
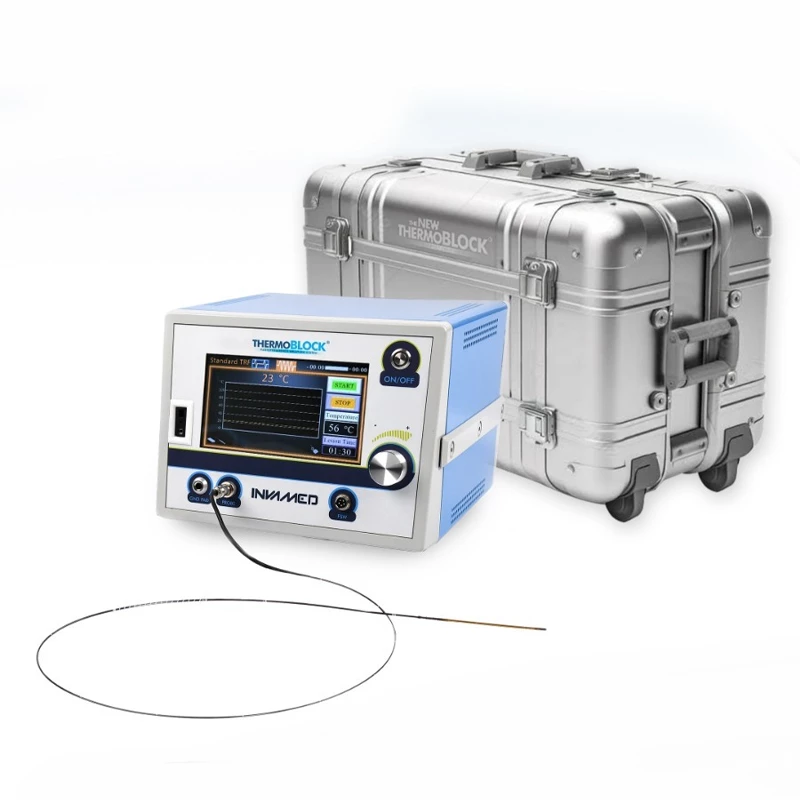
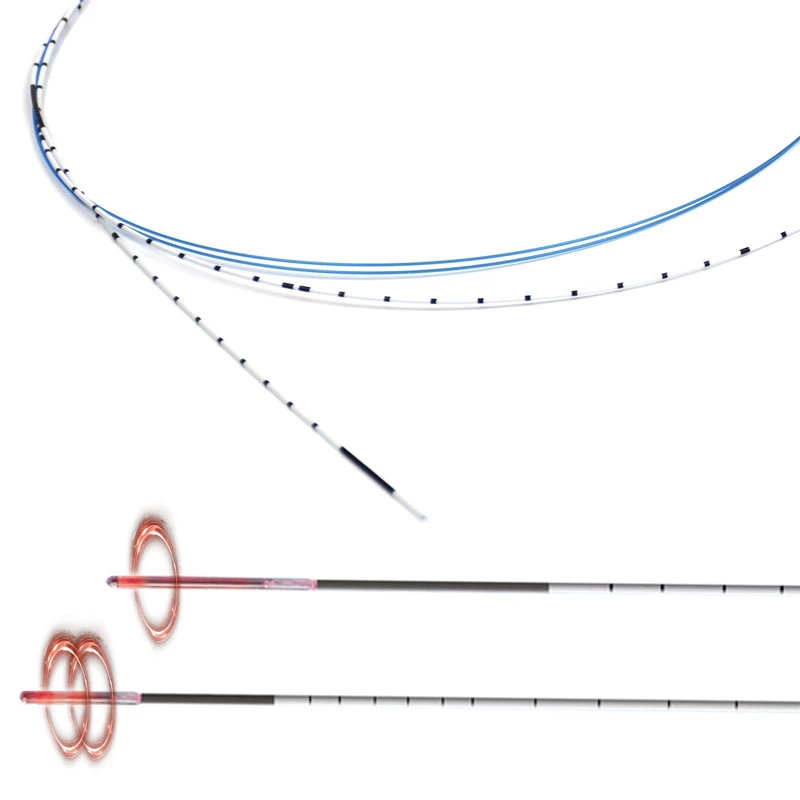
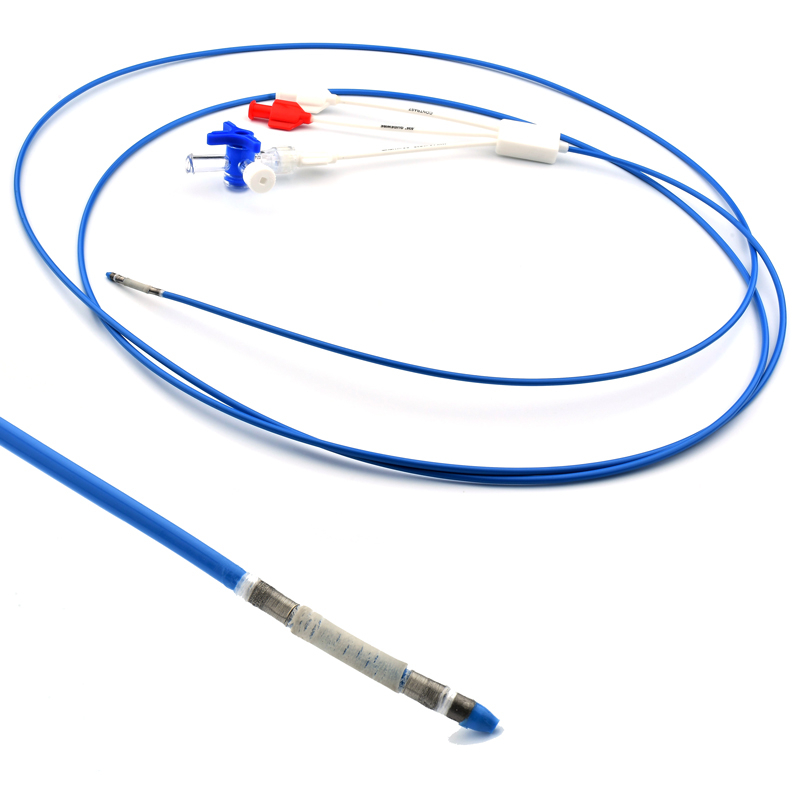
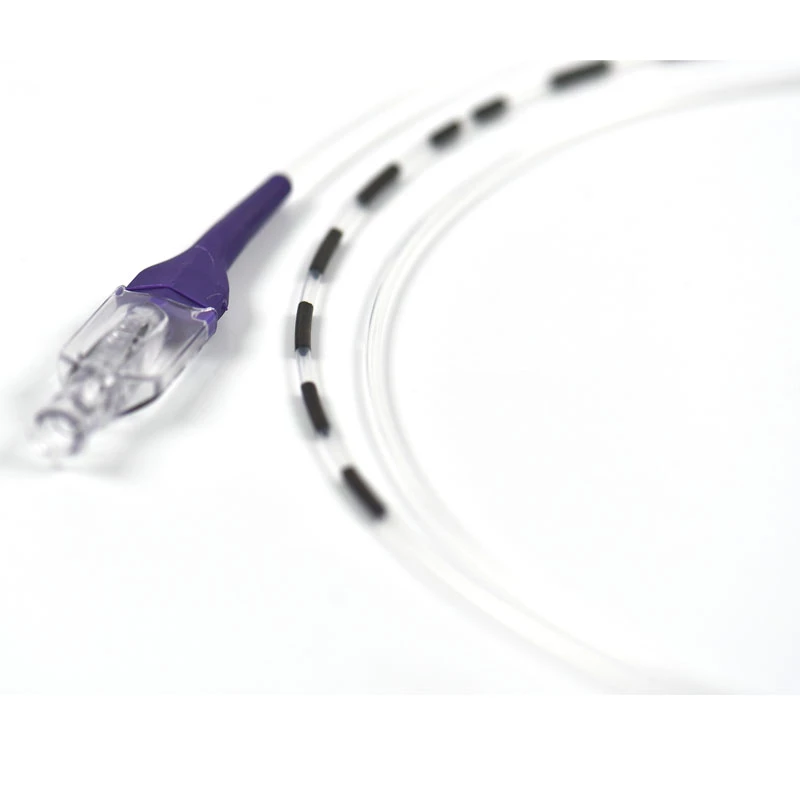
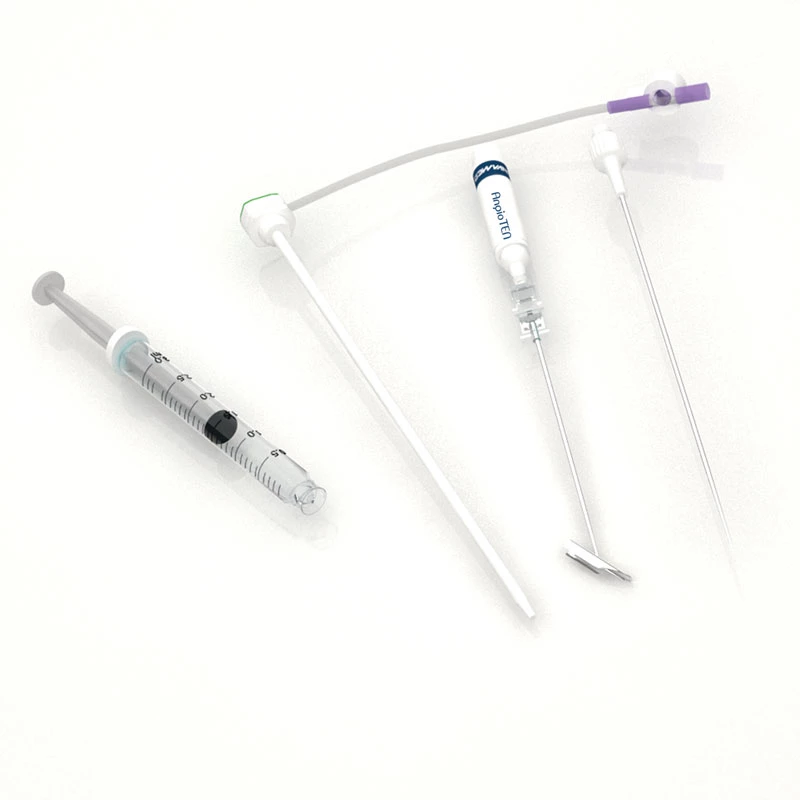
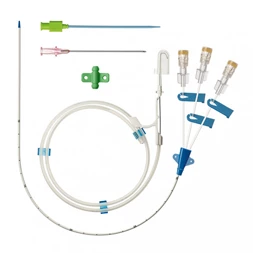
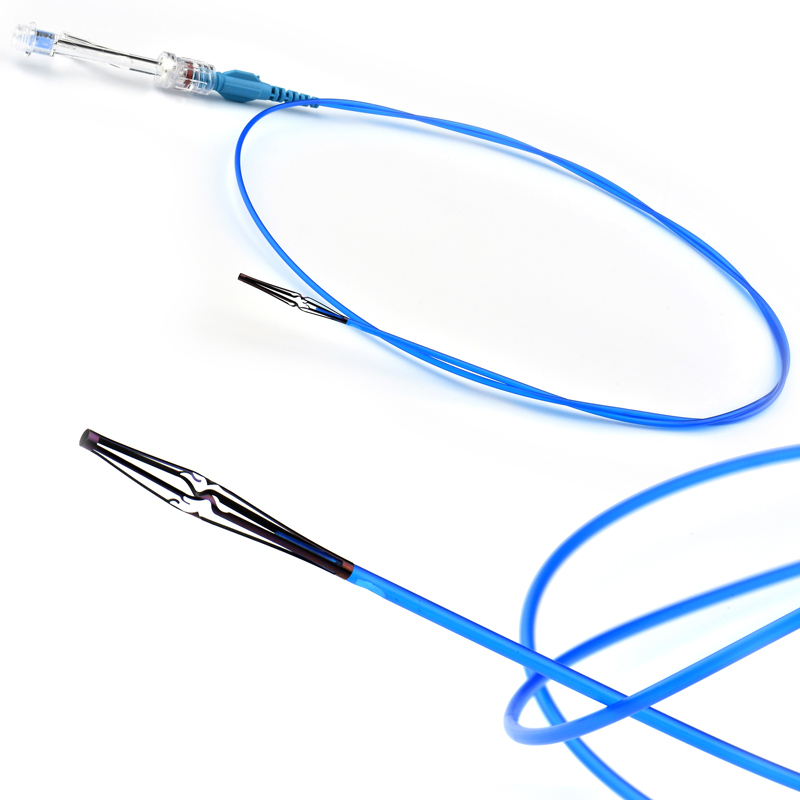
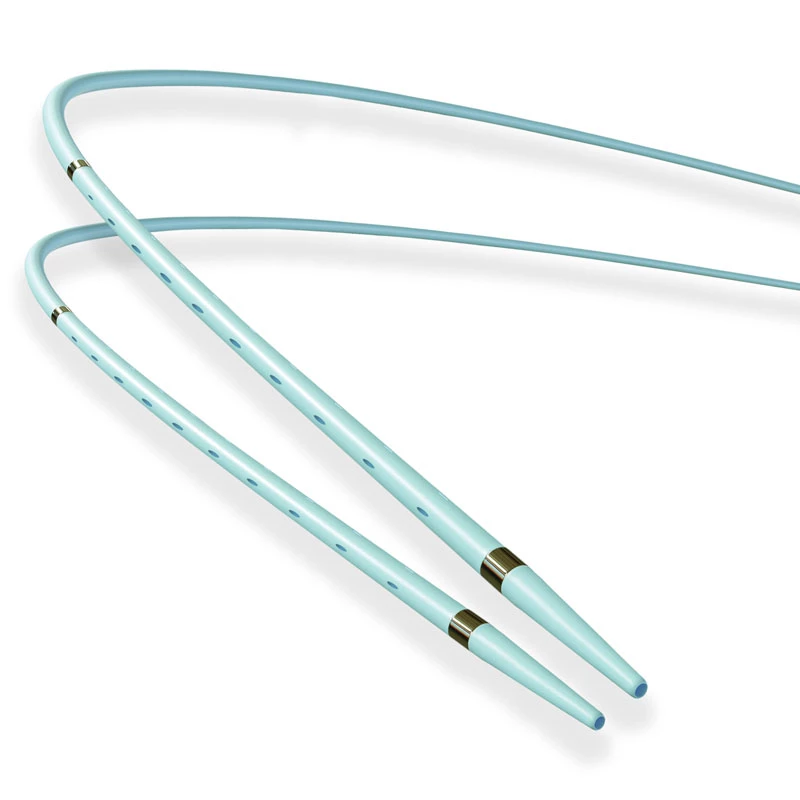
MicroCATH
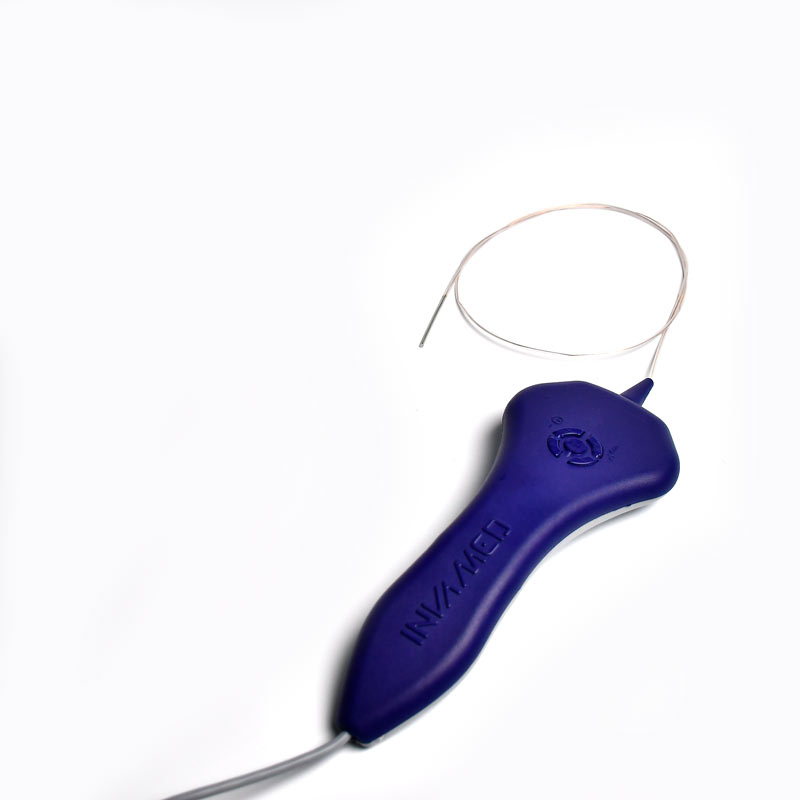
Flow Direct Catheter
MicroCATHⓇ Catheter is a Neurovascular intervention that uses minimally-invasive endovascular techniques to treat vascular diseases of the brain. Physicians who perform these procedures receive highly specialized training in interventional neuroradiology and/or endovascular neurosurgery.
| Usable Length | 90 cm, 150 cm | |
| Tip Length | 1.5 cm, 3 cm | |
| Tip Shape | Straight tip | |
| Catheter Profile Proximal | 2.1F, 2.7F | |
| Catheter Inner Diameter | 1.3F, 1.5F | |
| Radiopaque Marker | 1 mm located at 2 mm from the tip | |
| Guidewire Compatibility | Maximum diameter 0.012" | |
| Coating | Hydrophilic | |
| Minimum Dead Space | 0.2 ml | |
| Structure of the Catheter | PE/PEBAX |
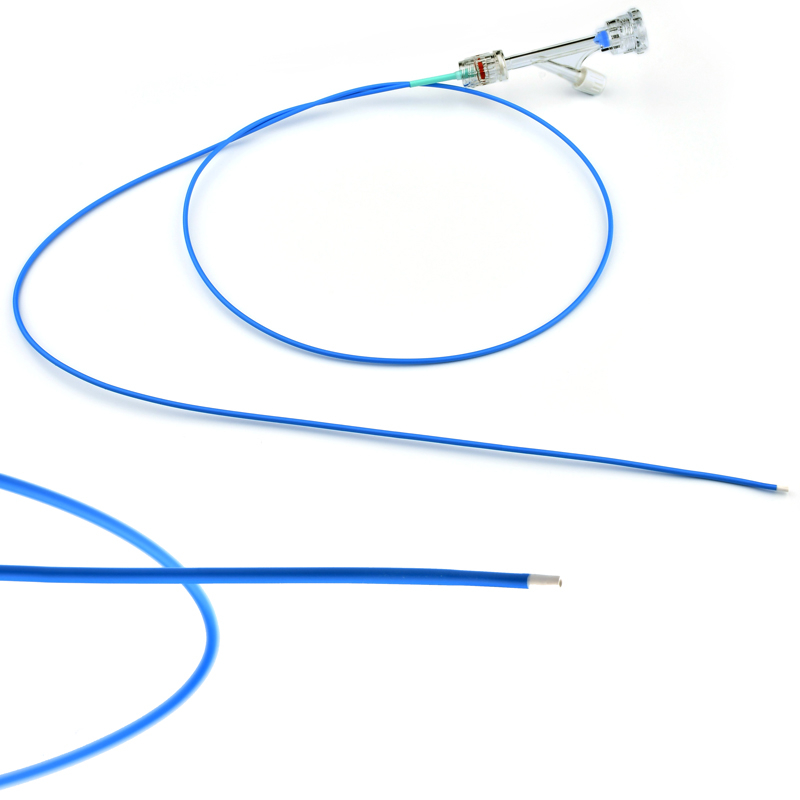
Invamed MicroCath Catheter products are Medical-Surgical products (which can be used inside the body in surgeries) with the qualifications required by MEDDEV 2.7.1 and the “EU Directive” numbered 93/42/EEC. There are different types according to different usage areas and diameters. It is suitable for the use of 0.014” micro guide wire and is supplied together. The tip has a radiopaque marker and is highly visible under ultrasound. 5F, 6F, and 7F diameter; It has 90 cm and 150 cm lengths and is compatible with embolizing agents. DMSO compatible. It has an internal diameter of 0.028 in (0.7 mm/0.6 mm/0.5 mm) flowing minimally in the distal regions. The microcatheter lumen can be contained within steerable guidewires 0.018 in (0.47 mm) in diameter or less. Embolizing agent is compatible.


Detailed specifications
We offer advanced solutions for the treatments of the following brain diseases:
- Intracerebral AVMs and AV Fistulas
- Intracerebral aneurysms
- Inntracranial stenosis
- Ischemic stroke and intracerebral thrombus
- Tumors
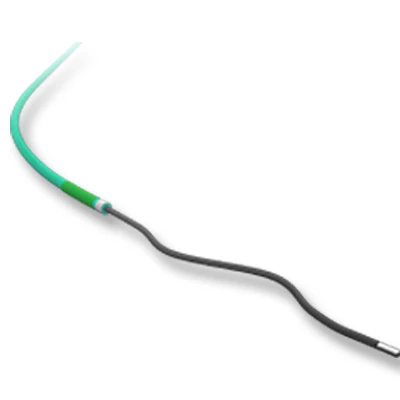
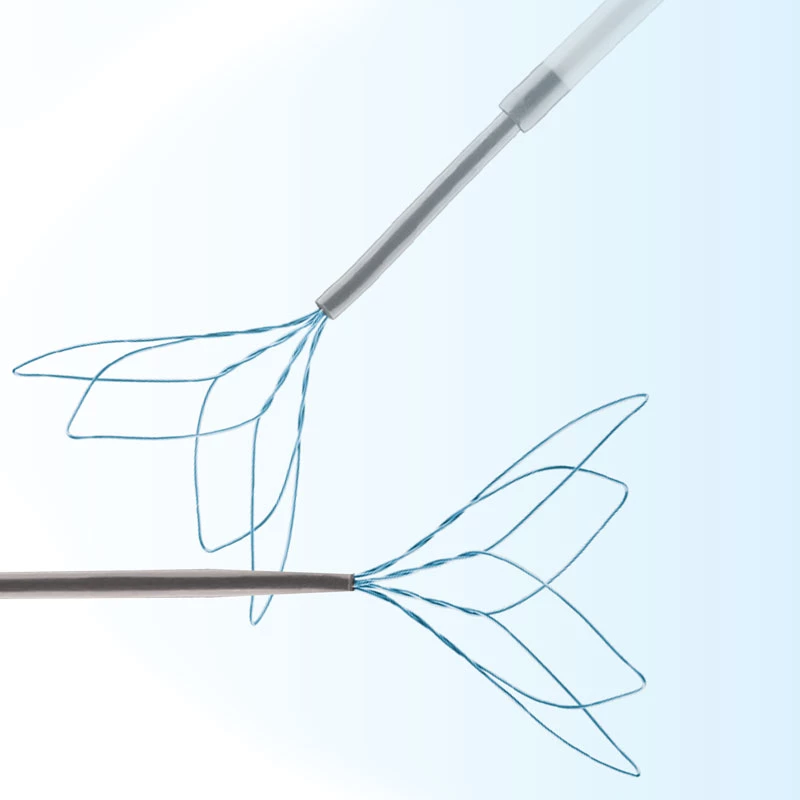
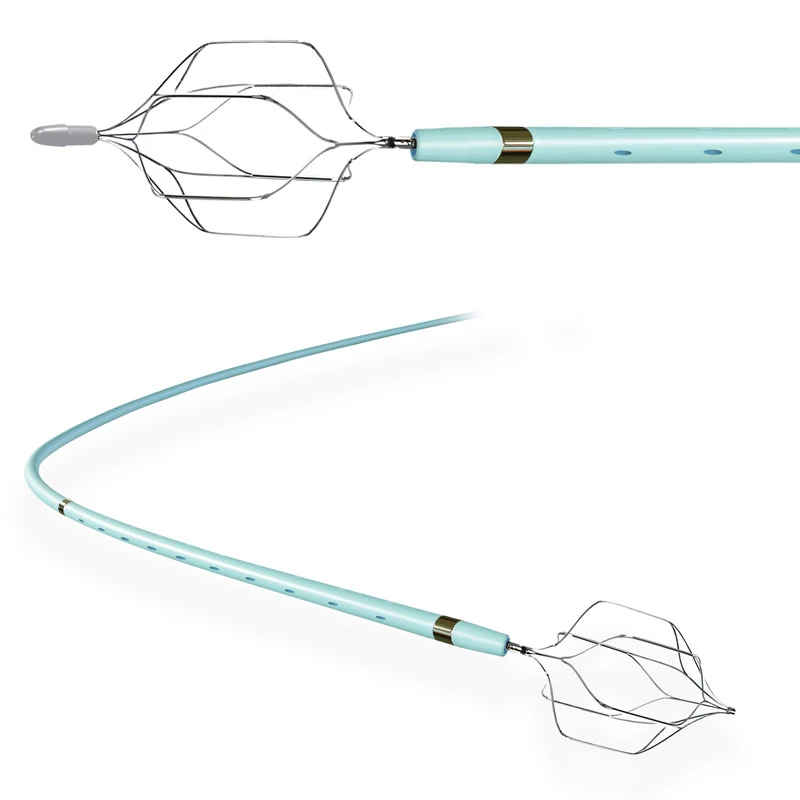
MicroCATHⓇCatheter is the Flow dependent microcatheter with a strong resistance to pressure and total DMSO compatibility.
The catheters are the only real flow-dependent catheters, meaning that their progression through the arteries system is facilitated by the blood flow. This characteristic is achieved thanks to an extreme suppleness of the tubes which allows a fast and non-traumatic progression of the catheter inside the blood vessels.
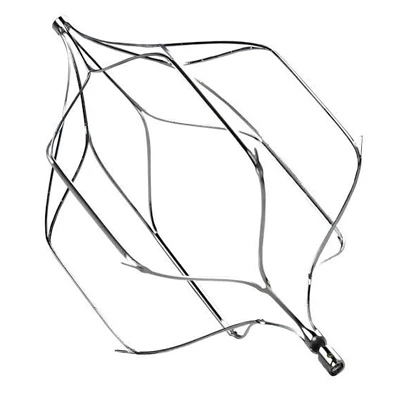
Detectable-part
Compatible embolizing agents:
- NBCA
- ethanol
- lipiodol
- microspheres
- PVA Particles
- Chemoembolization Agents
- Contrast media
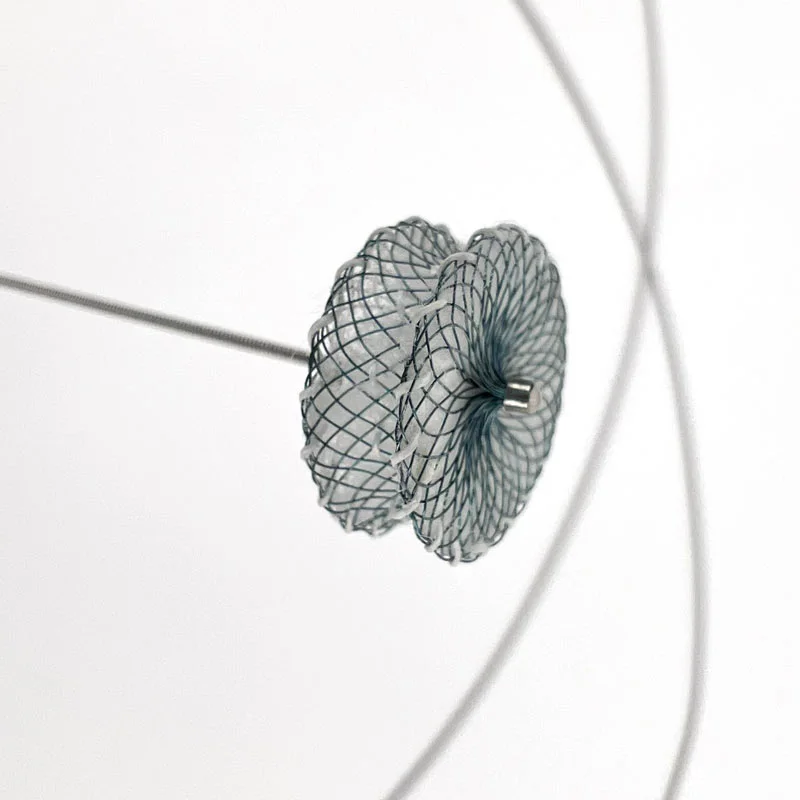
The use of the MicroCATHⓇ detachable-tip microcatheter for embolization of AVMs and AVFs is associated with high rates of successful catheterization and obliteration and low rates of morbidity and mortality.
- Hydrophilic coating
- Excellent kink resistance and pushability
- Dedicated tip design with radiopaque marker
- Excellent crossability
- Ensures reliable fluoroscopic visibility
- Choice of different catheter sizes ( 2.1 Fr and 2.7 Fr, guide catheter compatibility)
- Optimized tip design
- High compressive strength
- Low profile
- DMSO compatibility
- Embolizing agent compliance
- Flow Directed
- Detachable Tip structure
- Over the guidewire (0.012”) system avoiding vessel wall damage.
| Usable Length | 90 cm, 150 cm | |
| Tip Length | 1.5 cm, 3 cm | |
| Tip Shape | Straight tip | |
| Catheter Profile Proximal | 2.1F, 2.7F | |
| Catheter Inner Diameter | 1.3F, 1.5F | |
| Radiopaque Marker | 1 mm located at 2 mm from the tip | |
| Guidewire Compatibility | Maximum diameter 0.012" | |
| Coating | Hydrophilic | |
| Minimum Dead Space | 0.2 ml | |
| Structure of the Catheter | PE/PEBAX |
| Order Number | Proximal Outer Diameter | Inner Diameter | Distal Outer Diameter | Usable Lenght | Max Guidewire compatibility | Distal Tip Lenght |
| VS1168 | 2.7 Fr | I.D. ≥ 0.013" / 0.33 mm | 1.5 / 0.020” | 90 cm | 0,010” | 3 cm |
| VS1169 | 2.7 Fr | I.D. ≥ 0.013" / 0.33 mm | 1.5 / 0.020” | 150 cm | 0,010” | 1.5 cm |